Carbyne
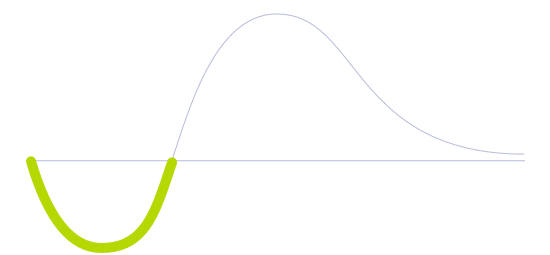
Technology Life Cycle
Initial phase where new technologies are conceptualized and developed. During this stage, technical viability is explored and initial prototypes may be created.
Technology Readiness Level (TRL)
Experimental analyses are no longer required as multiple component pieces are tested and validated altogether in a lab environment.
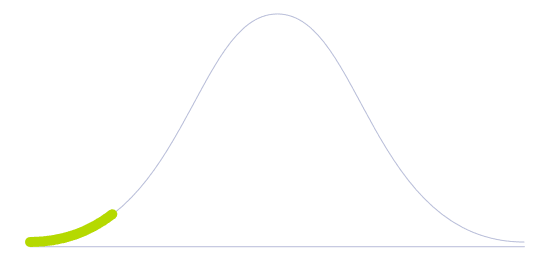
Technology Diffusion
First to adopt new technologies. They are willing to take risks and are crucial to the initial testing and development of new applications.

Carbyne is a linear acetylenic carbon molecule: an infinitely long carbon chain and is potentially the world's most robust material, with a tensile strength that is more than twice that of any other known material. Carbyne is also highly conductive, both thermally and electrically, making it useful in various applications.
In the field of electronics, for example, carbyne could be used to create more efficient and powerful electronic devices due to its high conductivity. It could also be used in the development of stronger and more lightweight materials for use in transportation, such as in the construction of aeroplanes or cars. Additionally, carbyne's exceptional strength and durability could be harnessed in the development of protective gear for military or law enforcement applications.
This new material is currently stable only when inside nanotubes and chain length are still limited. Furthermore, the molecules can be turned into a semiconductor when twisted. With unusual mechanical and electric properties, it has the potential to make nanomechanics, spintronic devices, and micro-electro-mechanical systems. It also has the potential for energy storage matrices like batteries and supercapacitors due to its extensive surface area in relation to mass, which is important for energy density.
Despite its many potential applications, carbyne is still in the early stages of development, and there are many challenges that must be overcome before it can be widely adopted. For example, it can be difficult to synthesize the material in a consistent and scalable way, and there are also concerns about its stability and reactivity when exposed to air or other elements.
Future Perspectives
One of the possible future applications of carbyne is the construction of spaceships and space elevators, which demand super-strong materials to form structures that could measure 65,000 kilometres and more. It could also make spacecraft bodies lighter and thus increase the power-to-weight ratio of these aerospace vehicles. By building a spaceship with carbyne, it could be possible to create a single-stage spacecraft that can reach orbit.
Image generated by Envisioning using Midjourney